

The Interfacial Character of Antibody Paratopes: Analysis of Antibody-Antigen Structures
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
We have studied for the role of interfacial water molecules that make
hydrogen bonds with both paratopes and epitopes, and suggested these water
molecules make important contributions to the interactions of antibodies and antigens.
Tyr, Ser, Asp, Asn make the highest numbers of hydrogen bonds with the interfacial
water molecules. From 403 antibody-antigen complexes, we have found the presence
of interfacial waters is found in 242 complexes, and suggested a compelling role of
these interfacial waters in the interactions of antibodies and antigens.
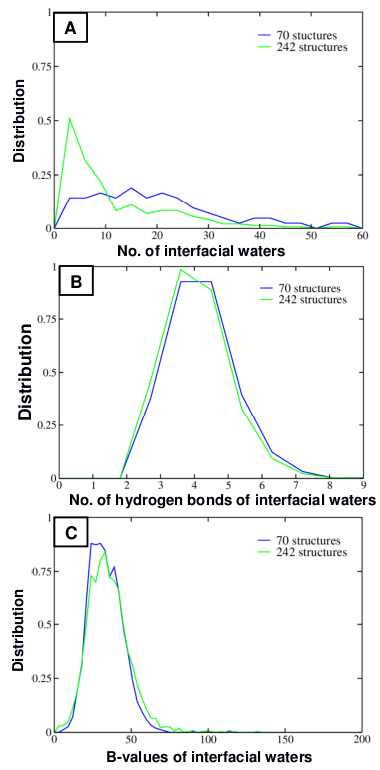
We identified 2580 interfacial water molecules from these 242 antibody-antigen complexes. The number of hydrogen bonds of contact residues with the 2580 interfacial water molecules is 3863. Compared to 3700 hydrogen bonds made by the all 8603 contact residues of antibodies with the antigens, this number suggests that the hydrogen bonds via interfacial water molecules contribute substantially in the interactions of antibodies and antigens. Among contact residues, Tyr, Ser, Asp, Asn make the highest numbers of hydrogen bonds with the interfacial water molecules, and a majority are through the sidechain atoms. In our study, we systematically mined the interfacial water molecules that were present at the interface of antigen and antibody. To account for the effect of the structure resolution on the number of water molecules resolved, we study the properties of interfacial waters in 2 datasets: (1) 70 high resolution structures with resolution less than 2 Å (average resolution = 1.81 Å) and (2) 242 structures (average resolution = 2.27 Å). The distribution of the number of interfacial water molecules (Figure 2A) shows that the structure resolution does indeed seem to affect the number of interfacial waters resolved and that more interfacial water molecules are observed in the dataset of 70 high resolution structures. Additionally, there is a high probability of interfacial waters mediating the epitope-paratope interaction. In most instances, there are 5-25 interfacial waters, and in a few cases >30 water molecules can be observed in these 70 antibody-antigen complexes. Furthermore, these interfacial waters engage in up to 3-6 interactions with other contact water molecules and contact residues of antibodies and antigens (Figure 2B). Additionally, these waters exhibit low B-values (Figure 2C). Together this suggests that these interfacial waters from the dataset of 70 high resolution structures are tightly bound at the interface and appear to enthalpically stabilize the complex. |
|
|
Figure 2: Interfacial waters observed in the antigen-antibody interface in a non- redundant dataset of 403 antigen-antibody complexes from PDB. (A) Distribution of the number of waters that were observed at the interface of antigen and antibody (B) Distribution of the number of hydrogen bonds made by these interfacial waters (C) Distribution of the B-value of these interfacial waters. |
|
|
|
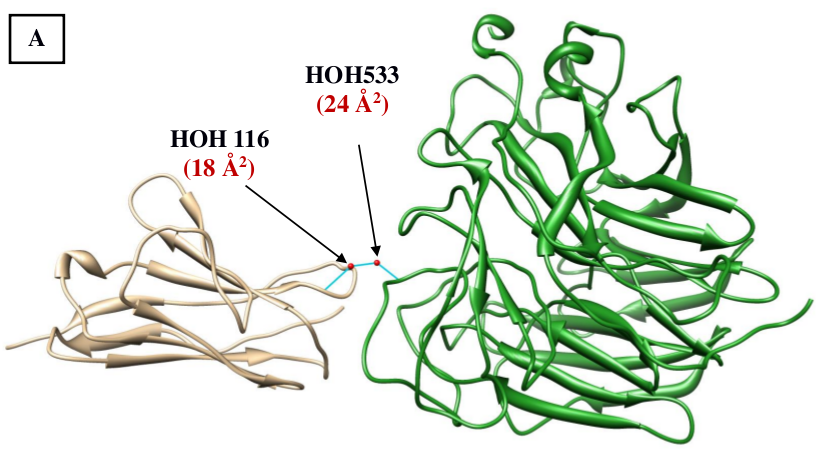
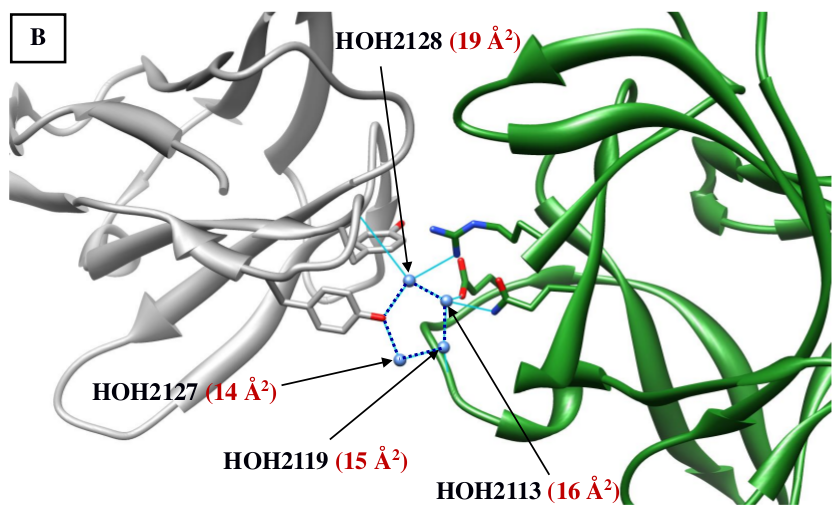
Figure 3: Higher ordered structures involving water molecules observed at the interface of antigen and antibody. (A) A water-bridge motif involving two water molecules is shown to be bridging between the light chain (chain L; brown) of the anti-neuraminidase antibody NC10 and the antigen, neuraminidase from influenza virus (chain N; green) (PDB code: 1NMB). (B) A pentamer composed of 4 water molecules and the side chain of Tyr96 of the human IL-18 complexed to Murine IGG 125-2H (PDB code: 2vxtLI). B- values of the respective waters are shown in brackets. |