

The Interfacial Character of Antibody Paratopes: Analysis of Antibody-Antigen Structures
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
We carry out all 296,835 pairwise 3D structure comparisons of 771 structures of contact residues and their interfacial water molecules from the dataset of 403 antibody-antigen complexes using our 3D structure comparison method CLICK. A heuristic clustering algorithm is used to obtain unique structural similarities amongst these contact residues, and found to separate into 368 different clusters that are the basis for a new database. These clusters can be used to identify structural motifs of contact residues of antibodies for epitope binding, which are important for protein engineering of therapeutic and diagnostic applications.
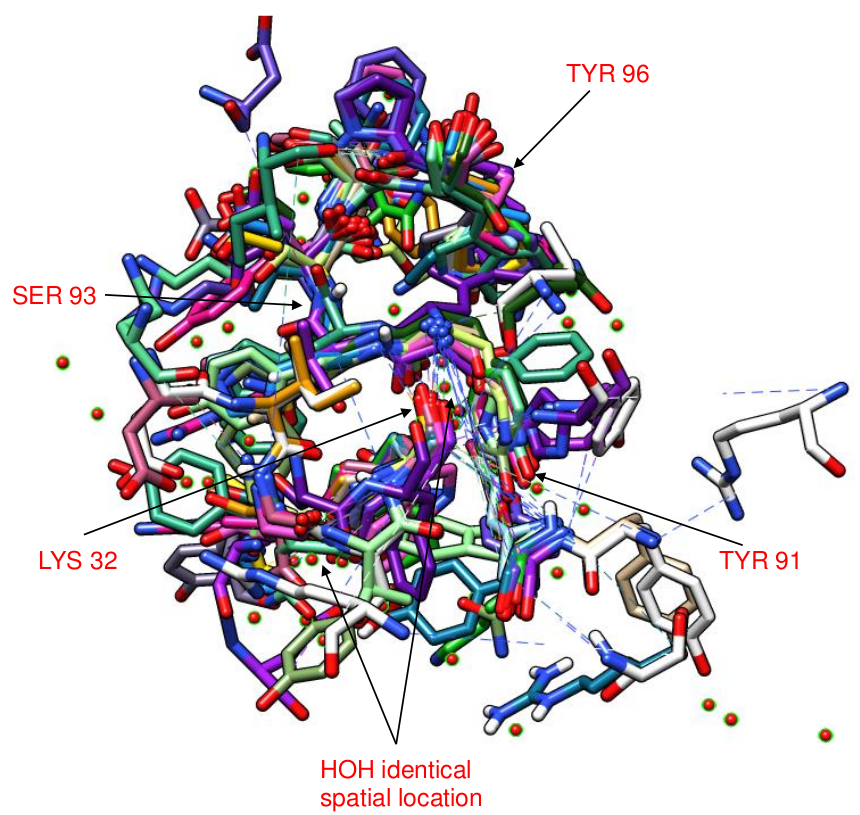
The list of structures in the 368 different clusters The detailed structures (PDB format) and the summary (.txt file) of each cluster from the 368 different clusters The largest cluster (cluster 29) has 27 structures, and the structure of 17 contact residues of the light chain of the human IL-18 complexed to Murine IGG 125-2H (PDB code: 2vxtLI) is the representative structure of this cluster. The CLICK superimpositions of the contact residues of light chain of 2vxtLI with the other contact residues of diverse antibody structures in this cluster help determine the common factors (structural motifs) in epitope binding. The CLICK superimpositions of the contact residues of light chain of 2vxtLI with the other contact residues of 26 antibody structures in this largest cluster confirm Tyr 91, Ser 93, Tyr 96 as well as Lys 32 of 2vxtLI to favour specific interactions in epitope binding as their aligned residues are in almost identical spatial locations (Figure 4). It is interesting to note that Lys 32, Tyr 91, Ser 93, Tyr 96 of 2vxtLI are mostly aligned with contact residues {Tyr, Asn), {Tyr, Ser, His}, {Ser, Glu}, {Tyr, Phe, Trp, Arg} from the other 26 antibody structures. Several interfacial water molecules of anitibody structures in this cluster are in almost identical spatial locations (Figure 4). Our analysis of this cluster suggests that CA and CB atoms of Lys 32, Tyr 91, Ser 93, Tyr 96 of 2vxtLI could be used as a structural motif for epitope binding. |
|
|
Figure 4: CLICK superimpositions of contact residues of 26 antibody structures with those of 2vxtLI in the largest cluster (cluster 29). There are 4 contact residues of 2vxtL: Lys 32, Tyr 91, Ser 93, Tyr 96 where their aligned residues are almost identical spatial location. |